A modified approach to harvesting stem cells is showing promise
Published 5:00 am Thursday, October 6, 2011
- A modified approach to harvesting stem cells is showing promise
Stem cell research has had many ups and downs, but a stream of setbacks had left researchers almost back at square one.
Now, researchers at the New York Stem Cell Foundation Laboratory have developed a method that may help recover the lost ground.
Embryonic stem cells can generate all the tissues of the body. So if such cells could be developed from a patient’s adult cells, it might be possible to make replacement cells for any diseased tissue without fear of rejection.
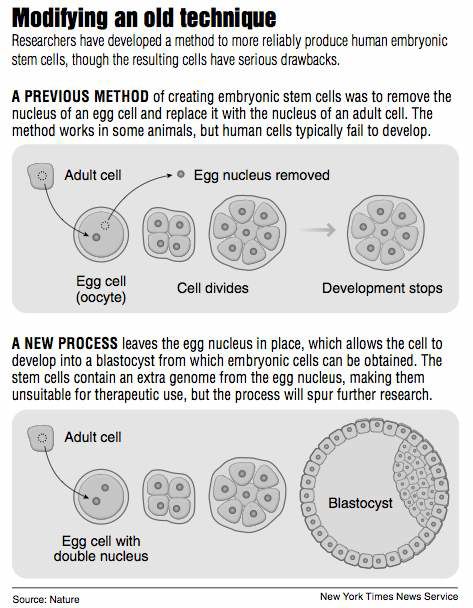
At first, it seemed that embryonic stem cells could be made by implanting the nucleus of a patient’s adult cell into an unfertilized human egg, or oocyte, whose nucleus had been removed. Mysterious factors in the body of the egg instruct the adult cell how to lose its specialist nature and revert to the embryonic state, where all fates are open to it.
But the method is highly inefficient and would require tens or hundreds of oocytes for each patient. A major advance was made in 2007 when Shinya Yamanaka found a way of avoiding all use of human oocytes.
He guessed how the oocyte reprograms the inserted nucleus, and showed that by injecting just four protein factors into a patient’s adult cell he could make it revert to the embryonic state. Cells made by Yamanaka’s method are called induced pluripotent stem cells, or iPS cells. At first they seemed identical, as hoped, to the embryonic cells made with the oocyte method.
But as researchers examined them more closely, they found more and more abnormalities, making the cells unsuitable for therapeutic use. In particular, iPS cells seem to retain a memory, in the form of chemical signals in the material that controls their DNA, of their previous identity. This memory trace would prevent them from morphing into other types of cell as required.
Going back to basics
A team led by Scott Noggle and Dieter Egli of the New York Stem Cell Foundation has now gone back to the basics by trying to improve on the original method of using oocytes to generate patient-derived embryonic stem cells.
By a lucky accident as part of a control experiment, they left an oocyte’s nucleus in place when they implanted an adult nucleus. They noticed that in the presence of the oocyte nucleus, embryos with an inserted adult cell nucleus developed much further than usual. They progressed, in fact, to the blastocyst stage, the point at which embryonic stem cells can be harvested, the researchers report in the Wednesday issue of Nature.
With several species, including mice or cows, the cells easily reach the blastocyst stage, and from there the donor can be cloned when the blastocyst is implanted in a uterus. But despite all the worry about cloning humans, it has in fact been almost impossible to coax human oocytes with an implanted nucleus to progress to the blastocyst stage. The New York researchers say they have been unable to repeat the one such claim that has been published so far.
The blastocysts produced by the New York method are triploid, meaning they have the normal diploid, or double genome, of the inserted adult cell plus the single genome of the oocyte. Triploid cells are unstable and possibly cancerous and could never be injected into patients. Moreover, the process is too inefficient for therapeutic use — 63 oocytes were required to generate one normal set of embryonic cells.
“These cells are not therapeutically relevant at the moment,” Noggle said.
Their relevance is for research, to study the process of blastocyst development and in particular to explore why the iPS cells produced by Yamanaka’s method are imperfectly reprogrammed. It may be that other factors are required besides the four identified by Yamanaka. If these were to be identified, the iPS cells could be put back on track as a starting point for therapy. There would still remain many difficult steps, like ensuring that cells that are generated to repair the patient’s tissue are correctly programmed, well behaved and noncancerous.
‘A stepping-stone toward success’
Noggle and Egli also hope to adapt their method to produce usable patient-derived blastocysts. If the human oocyte nucleus is taken out too early, it apparently fails to provide enough of the factors needed to reprogram the nucleus. If it is left in beyond the first cell division, it fuses with the inserted nucleus and cannot be removed. It may be possible to yank it out at the last possible minute, when it has done a sufficient reprogramming job, and obtain a normal diploid blastocyst formed from the patient’s own cells.
The research “stands as a stepping-stone toward success,” Dr. George Daley, a stem cell expert at Children’s Hospital Boston, writes in a commentary in Nature. It also raises the “provocative question,” he says, of whether patient-derived embryonic cells made the old-fashioned way with oocytes might perform better than iPS cells.






